Pioneers in Neurotechnology
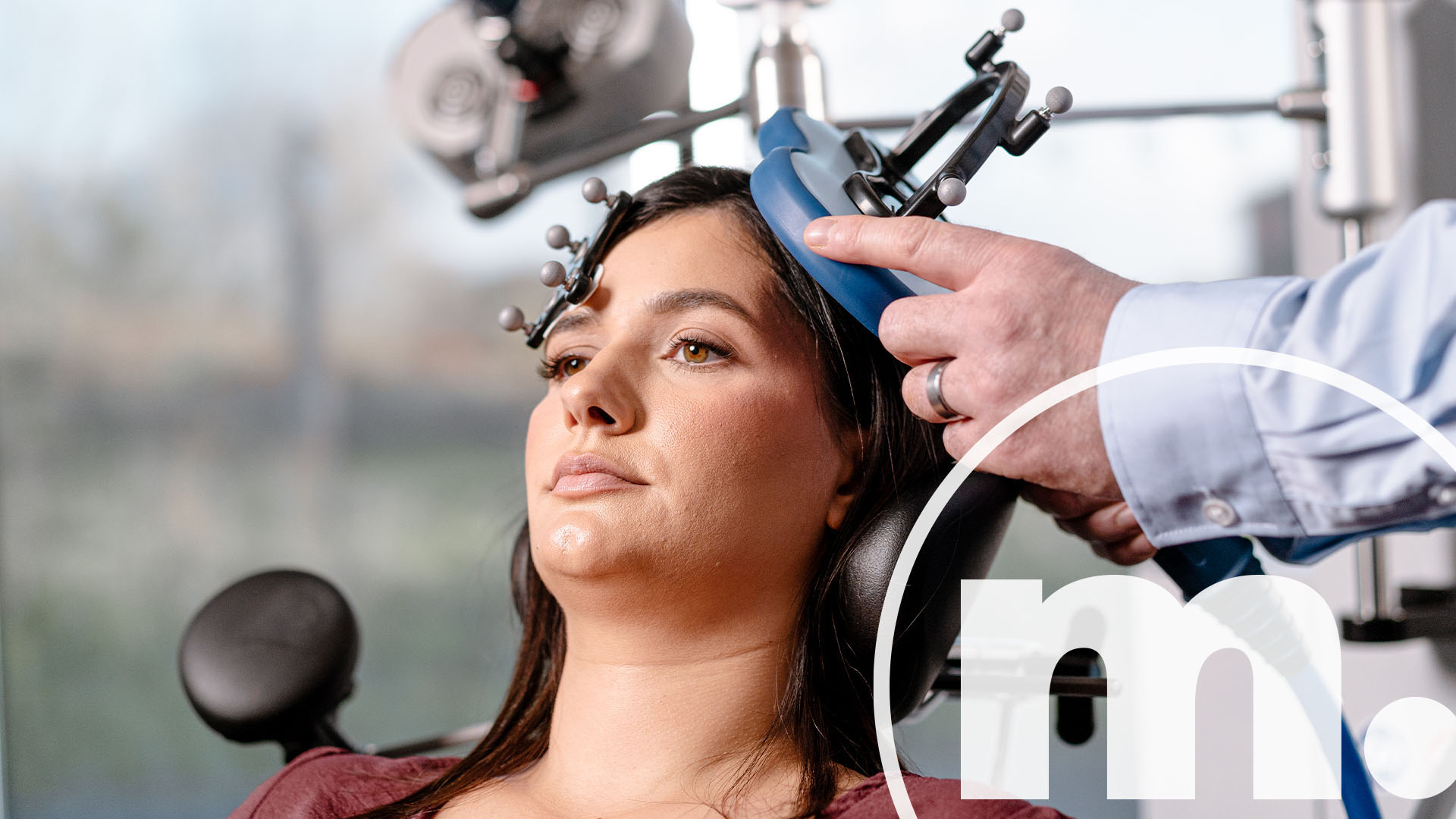
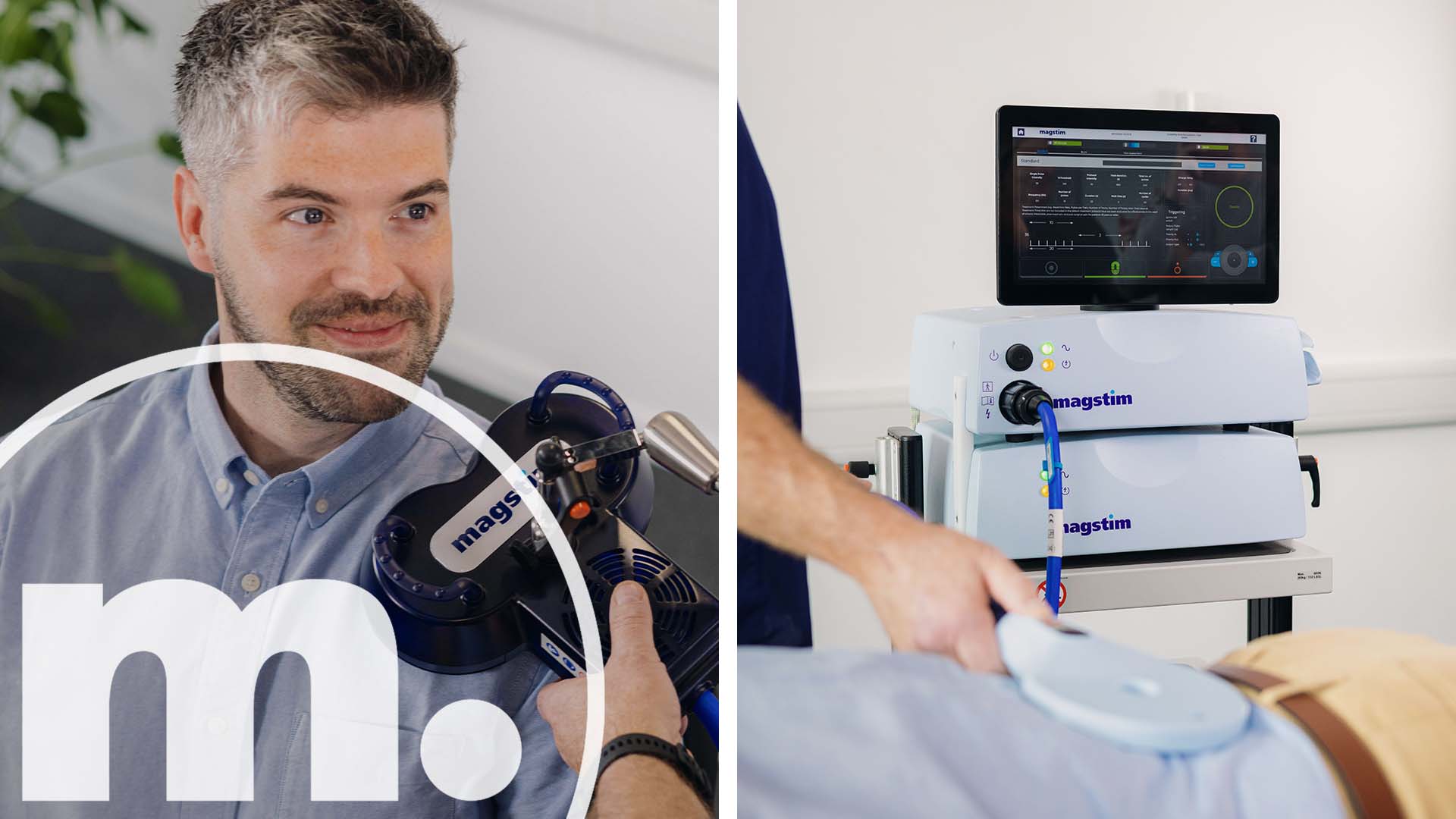
Welcome to Magstim, your neurotechnology partner for EGI Electroencephalography (EEG), Transcranial Magnetic Stimulation (TMS), Transcranial Direct Current Stimulation (tDCS) and other multi-modality integrated solutions. With a rich history spanning four decades, Magstim has been at the forefront of innovation in neurotechnology.
Magstim is committed to developing advanced solutions to study and treat the brain. Our technologies provides neuroscience researchers and clinicians with tools to make impactful change in global healthcare.
From our pioneering TMS therapy systems to our advanced EGI HD-EEG solutions and portable tDCS devices, Magstim delivers unparalleled precision, reliability, and efficacy, empowering professionals to make breakthroughs in brain science and improve patient outcomes.
Backed by a global team of experts with extensive knowledge and experience in neuroscience and neurostimulation, Magstim is dedicated to providing exceptional customer support and service, ensuring customers have the resources they need to accelerate research and care for future generations.
Feedback


An ingenious way to apply a large number of electrodes in minutes. The application is fast, painless, reduces anxiety about the experience, and thereby increases compliance.


I have been fascinated by the incredible responses that we have had from patients. It has done wonders for so many of them.


After three weeks of TMS, I was sleeping less during the day, becoming more involved in my family and their lives again. In addition, I’ve been successful at tapering down my antidepressants to less than a fourth my original dose.


An ingenious way to apply a large number of electrodes in minutes. The application is fast, painless, reduces anxiety about the experience, and thereby increases compliance.


I have been fascinated by the incredible responses that we have had from patients. It has done wonders for so many of them.


After three weeks of TMS, I was sleeping less during the day, becoming more involved in my family and their lives again. In addition, I’ve been successful at tapering down my antidepressants to less than a fourth my original dose.