The New Magstim Rapid
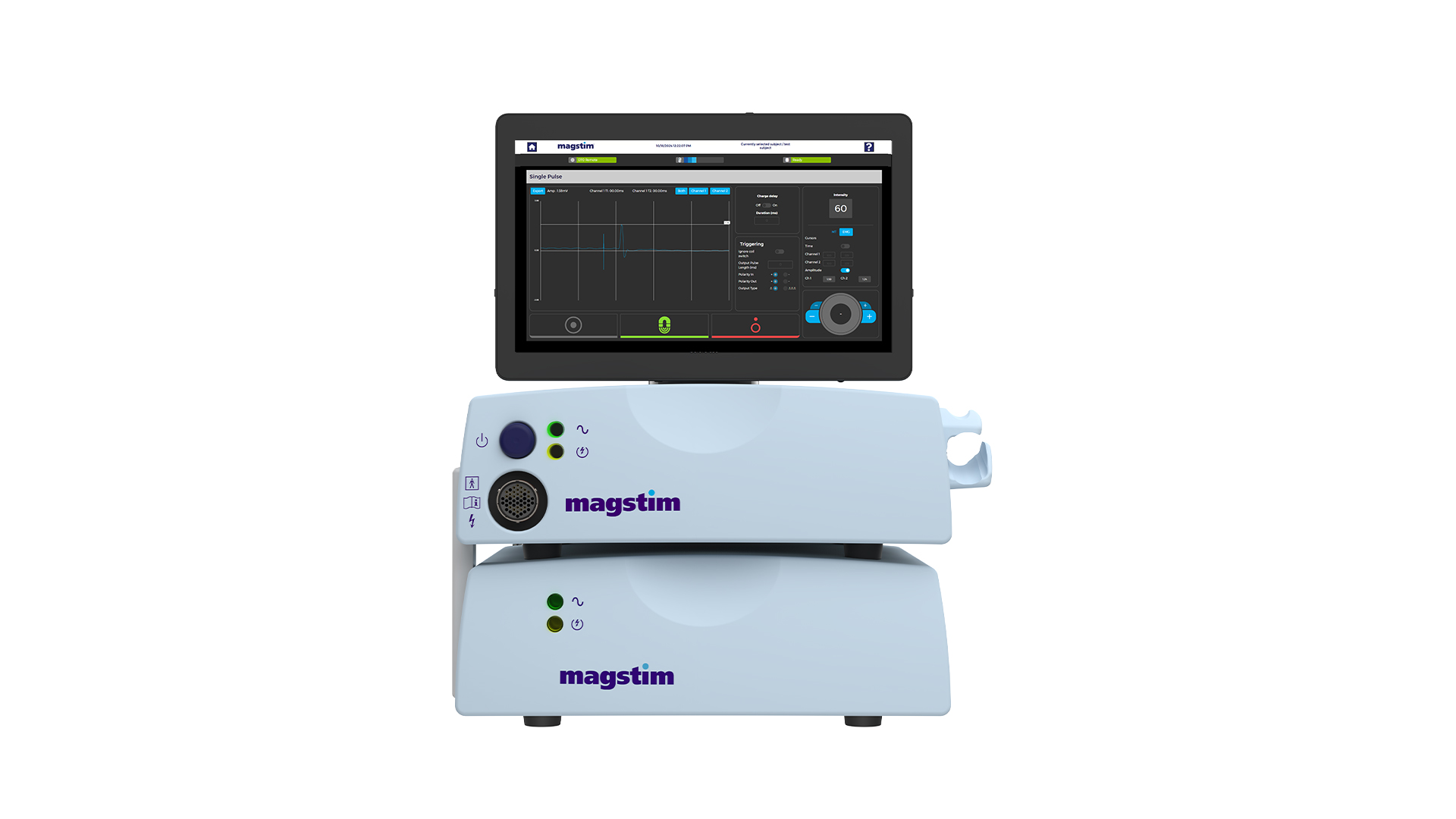
At Magstim, innovation never stands still—and neither should your research. With the launch of the original Rapid in 2004, we’re proud to announce the release of the New Magstim Rapid rTMS System, a powerful update to one of the most trusted TMS devices in neuroscience.
Engineered with enhanced performance, improved usability, and comprehensive integration capabilities, this next generation device is your gateway to new possibilities in transcranial magnetic stimulation (TMS) research. The updated Rapid rTMS System includes a range of new features designed with researchers and medical professionals in mind.
Magstim Rapid stimulators have been used in numerous fundamental and clinical research studies worldwide, offering performance capabilities to meet a variety of research requirements. The Rapid series is compatible with a wide range of Magstim coils.
Other features include a sleek, upgraded 15” touchscreen, streamlined patient data management tools and user-friendly software, which offers an intuitive experience, while a Windows-based platform enables greater compatibility and external integrations. Whether you’re conducting brain research, studying pain pathways, or integrating with EMG, EEG, or TMS-evoked potentials, the new Rapid provides the tools you need to push boundaries and uncover new insights.
For availability in your territory, please speak to your local Magstim representative. Subject to territory registrations.

Magstim TMS for Pain Relief
Magstim is proud to announce that the Rapid rTMS System is now FDA cleared for the stimulation of peripheral nerves in pain relief*, opening the door to expanded treatment possibilities for patients experiencing chronic pain. This clearance represents a major step forward in the field of non-invasive neuromodulation, offering medical professionals a powerful new tool grounded in scientific research and clinical efficacy. By targeting peripheral nerves, the system provides an alternative pain relief approach that avoids the need for pharmaceuticals or invasive procedures.
This new capability reinforces Magstim’s commitment to advancing neuromodulation and supporting healthcare professionals in transforming patient care. To speak to a Magstim Expert, please contact us.
*FDA Cleared for the stimulation of peripheral nerves for relief of chronic intractable, post-traumatic, and post-surgical pain for patients 18 and older.
System Configurations
Choose from a range of 3 configurations

Unlock New Possibilities
Magstim’s Rapid series has long been a go-to choice for researchers across the globe, cited in thousands of peer reviewed studies. Now, with enhanced configurability, performance, and connectivity, this latest system ensures you stay at the forefront of neuromodulation technology.
Ideal for a wide range of fundamental and clinical research applications applying neural modulation.
Suitable for both cortical and peripheral stimulation.
Multiple configurations: Standard Rapid (50Hz), Super Rapid (100Hz), and Super Rapid Plus1 (100Hz).
Optional extras: TMS cart, treatment chair, and a variety of standard and sham coils.
Stimulator pulse configurations include: Biphasic, repetitive, standard and burst stimulation.
To learn more about the new Rapid, please join our webinar on the 30th July 12PM EST.