New Rapid
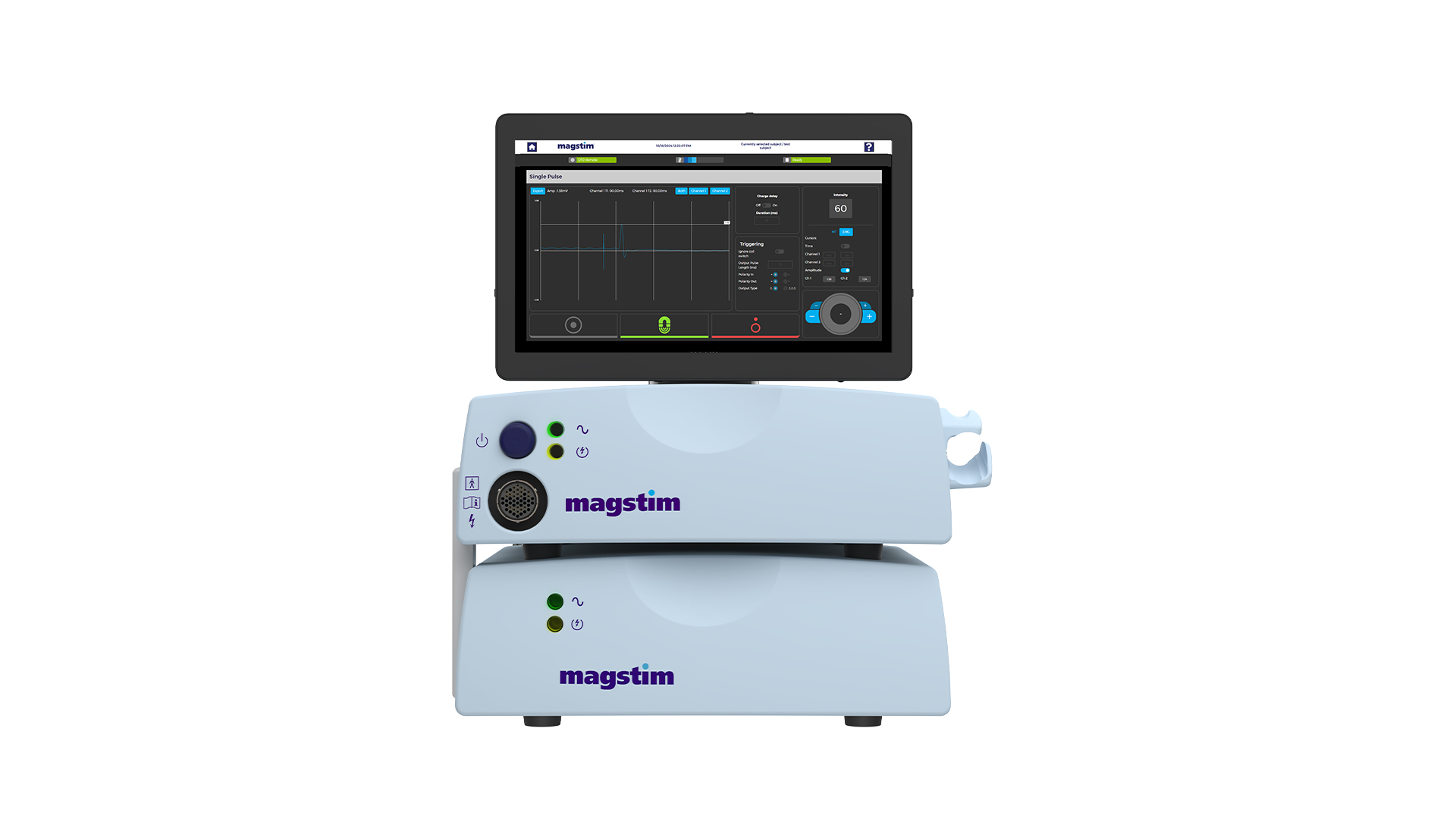
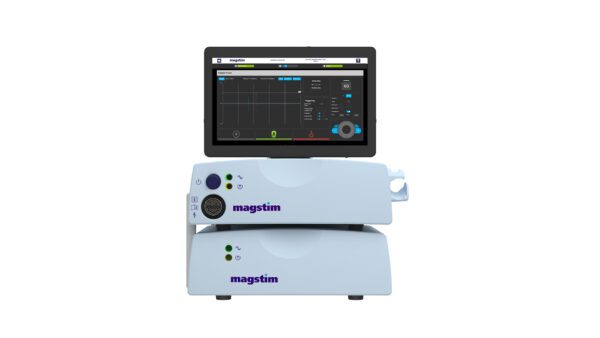
Unlock new possibilities with the New Magstim Rapid TMS system — your gateway to groundbreaking discoveries. The new updated Rapid TMS System includes a range of new features designed with researchers in mind. A sleek, upgraded 15” touchscreen and upgraded user-friendly software offer an intuitive experience, while a Windows-based platform enables greater compatibility and external integrations. Whether you’re conducting brain research, studying pain pathways, or integrating with EMG, EEG, or TMS-evoked potentials, the new Rapid provides the tools you need to push boundaries and uncover new insights.
Now FDA cleared for stimulation of peripheral nerves in pain relief, medical professionals can unlock new potentials for the treatment of pain in patients*.
Find Out More – Magstim Pain Therapy
Magstim Rapid stimulators have been used in numerous fundamental and clinical research studies worldwide, offering performance capabilities to meet a variety of research requirements, and compatible with a wide range of Magstim coils.
Capable of high-frequency and theta burst stimulation protocols for both cortical and peripheral research applications. Improved 2-channel EMG for motor evoked potential assessments (MEPs), with charge delay function for reduced artifacts during MEP or EEG recording. The new Magstim Rapid has integrated external triggering connectivity and integrated patient data management software tools.
Ideal for a wide range of fundamental and clinical research applications applying neural modulation. Suitable for both cortical and peripheral stimulation.
Specifications
Chronic pain remains one of the most significant challenges in modern healthcare – impacting mobility, emotional wellbeing, and overall quality of life. For millions of people, existing treatments such as medication, injections, or surgery often fall short of providing effective relief.
Today, there’s a new option changing the conversation around pain care: The Magstim Rapid – now FDA cleared for the treatment of chronic pain.*
Find Out More – Magstim Pain Therapy
*USA: Magstim Rapid systems are cleared for the stimulation of peripheral nerves for relief of chronic intractable, post-traumatic and post-surgical pain for patients 18 years or older. Pain Therapy with Magstim Rapid is subject to territory registrations.
- FDA cleared for stimulation of peripheral nerves in pain relief
- NEW 15” touchscreen for a modern, responsive interface
- Windows-based platform for enhanced external integration
- Improved MEP functionality for superior signal quality
- Streamlined patient data management tools
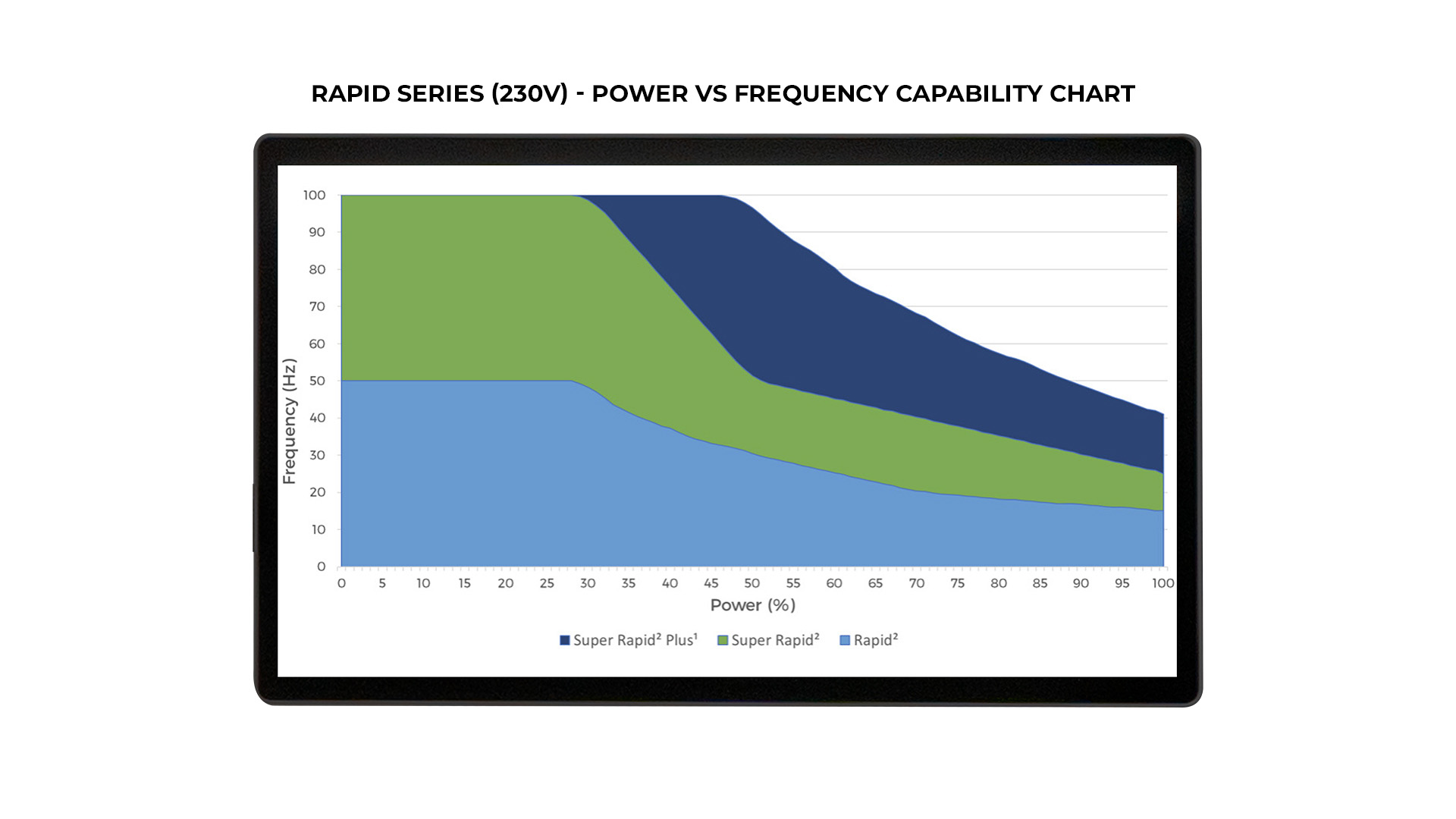
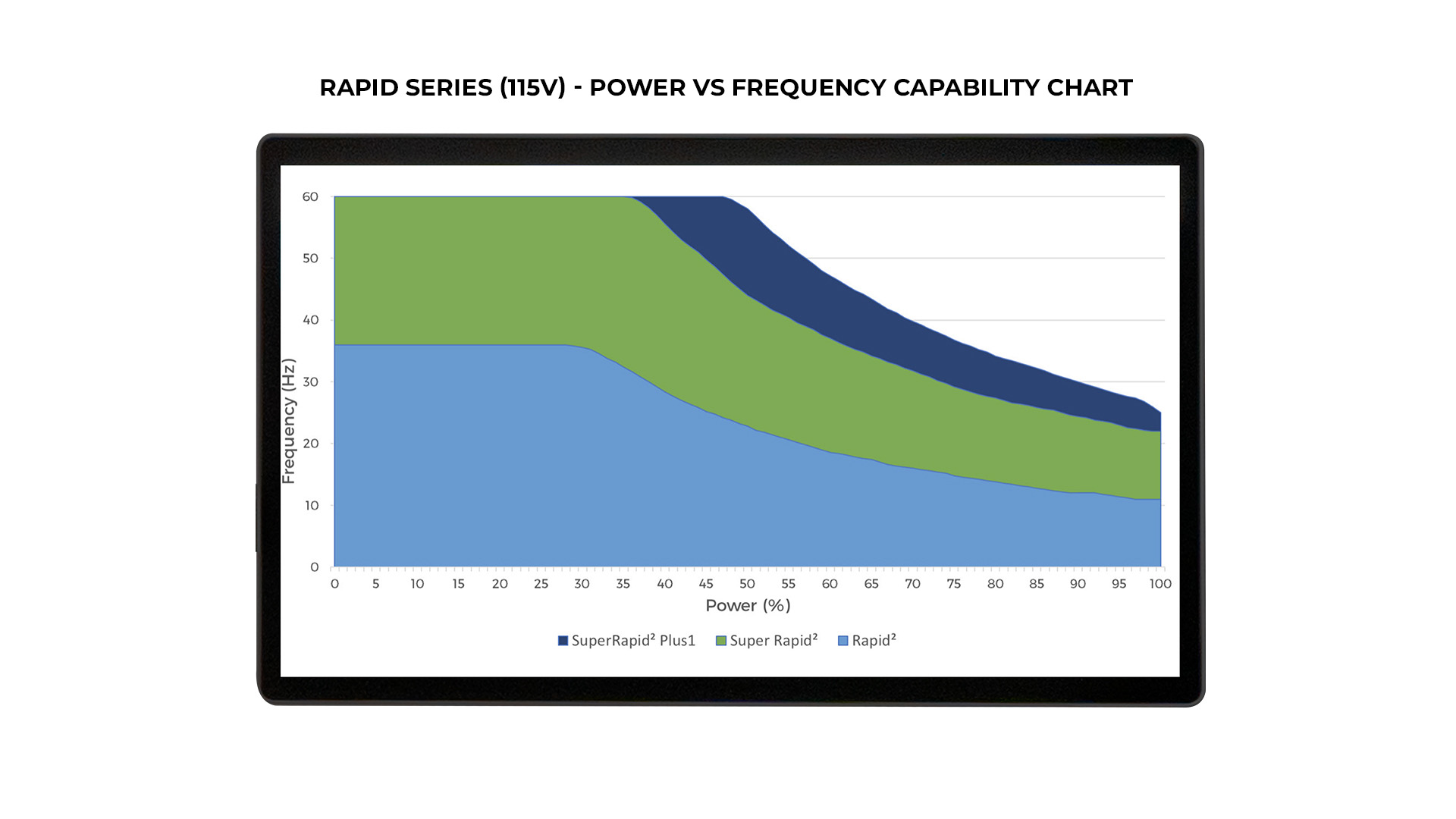
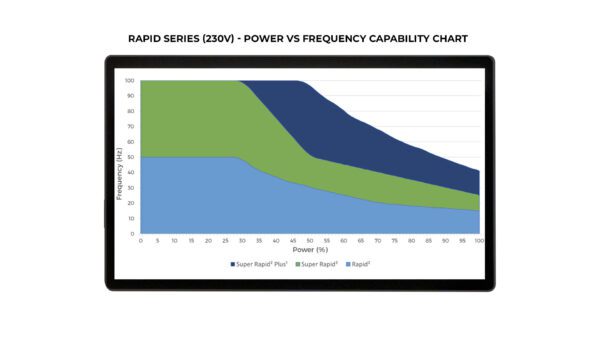
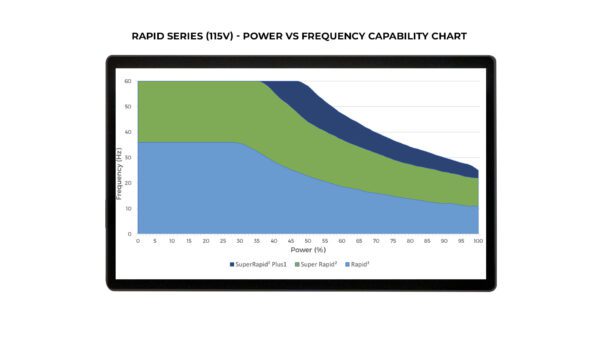
- Multiple configurations: Standard Rapid (50Hz), Super Rapid (100Hz), and Super Rapid Plus1 (100Hz) in both 115 and 230V
- Optional extras: TMS cart, treatment chair, and a variety of standard and sham coils
- Stimulator pulse configurations include: Biphasic, repetitive, standard and burst stimulation
Magstim’s Rapid series has long been a go-to choice for researchers across the globe, cited in thousands of peer-reviewed studies. Now, with enhanced configurability, performance, and connectivity, this latest system ensures you stay at the forefront of neuromodulation technology.
View Supporting Publications:
(PDF) The complex landscape of TMS devices: A brief overview
Research information Number of published articles for each commercial… | Download Scientific Diagram
FDA Cleared for the stimulation of peripheral nerves for relief of chronic intractable, post-traumatic, and post-surgical pain for patients 18 and older.
For a full list of regulatory clearances, please click here.
Part No: 6000-00: 230V (3kVA)
Part No: 6000-US : 115V (2.3kVA)
Each Rapid system configuration (Standard/Super/Plus1) has different capabilities with regards to Maximum Pulse Frequency vs Maximum System Power – see table images cited above.
Contact Us