Provider Spotlight
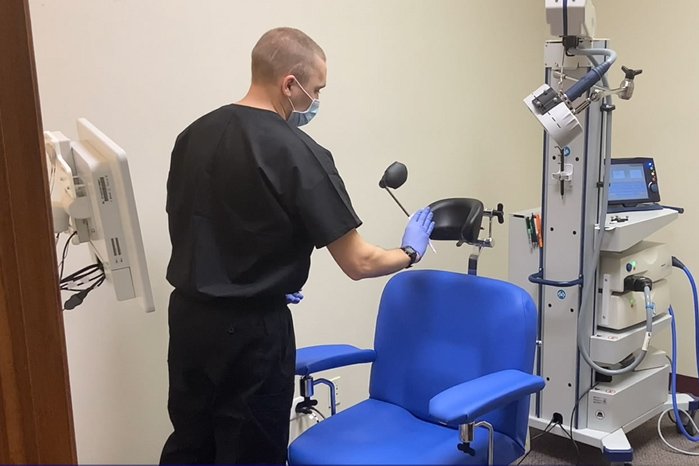
Thank you to our practitioners who continue to provide life-changing TMS treatments to their patients during the COVID-19 crisis.
In accordance with guidelines recommended by the CDC and other health organizations, many TMS treatment clinics are still operational. This is an example of a TMS center that continues to operate and support both existing and new patients.
In this video, Dr Aimee Harris-Newon from the TMS Center for Integrative and Functional Health and Wellness, outlines the operational procedures they have put in place in response to the COVID-19 pandemic.
Magstim TMS Therapy is FDA 510(k) cleared for the treatment of Major Depressive Disorder in adult patients who have failed to achieve satisfactory improvement from prior antidepressant medication in the current episode.


